Introduction:
In this experiment we are going to talk about vapor pressure and intermolecular forces. We are going to concentrate in butyl acetate (C6H12O2) and we are going to show a molecule of this organic compound and the different parts the compound has. We also going to show a graph where we increase the temperature of butyl acetate to see how does the pressure change. At the end we can see a table of results with the boiling points, the temperature and the pressure of seven different compounds.
Objectives in this lab session:
In this experiment we are going to talk about vapor pressure and intermolecular forces. We are going to concentrate in butyl acetate (C6H12O2) and we are going to show a molecule of this organic compound and the different parts the compound has. We also going to show a graph where we increase the temperature of butyl acetate to see how does the pressure change. At the end we can see a table of results with the boiling points, the temperature and the pressure of seven different compounds.
Objectives in this lab session:
1.To improve practical skills – use
of Schlenk tube, pressure sensors, vacuum line.
2.To investigate the structure and properties of one particular chemical.
3.To investigate the effect of
temperature on vapour pressure.
4.To
compare results with other groups (with other chemicals)
and relate them to
“intermolecular forces”.
Butyl
Acetate
Butyl Acetate is an organic
compound commonly used as a solvent in the production of lacquers (cosmetic)
and other products. It is a colorless flammable liquid. It is also found in
many types of fruit, it gives a sweet smell of banana or apple. It is used as a
synthetic fruit additive in foods such as candy, ice cream, cheeses, and baked
goods.
Molecular
formula: C6H12O2
Molecular mass: 116.16 g
mol-1
Boiling point: 127 °C; 261
°F; 400 K
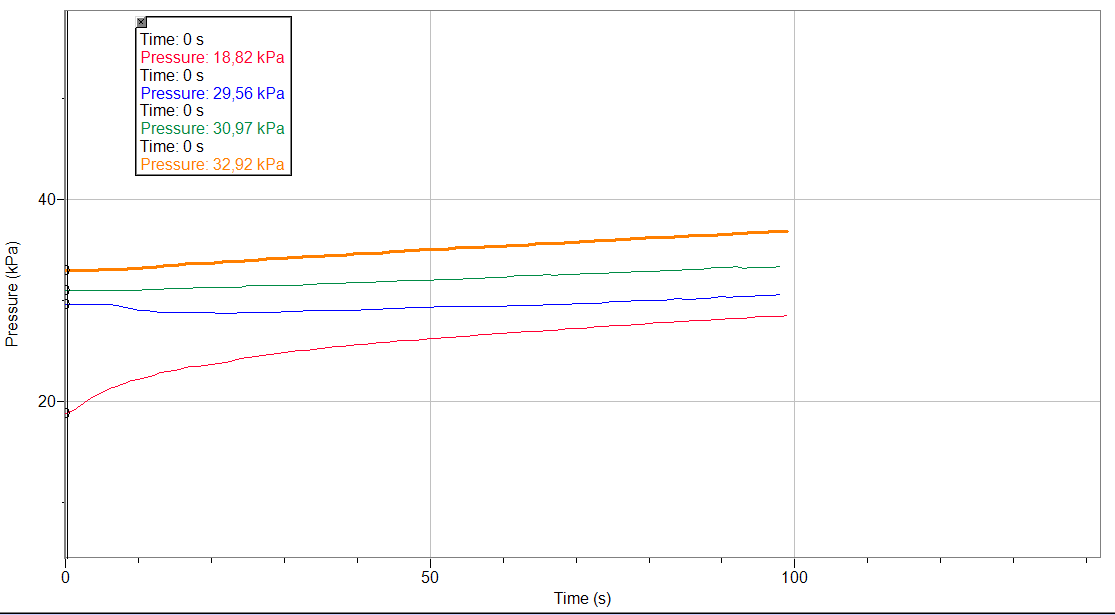
Graph:
Variation of pressure related to the time
Variation of pressure related to the time
Results:
Group
|
Chemical
|
Formula
|
Boiling point
|
Temperature and vapour pressure
|
1
|
Butyl Accetate
|
C6H12O2
|
20 ºC
|
0 ºC= 35,31 kPa // 15 ºC= 32,26 kPa // 20 ºC= 29,41 kPa // 40 ºC= 26,69 kPa
|
2
|
Diethyl Ether
|
(C2H5)2C
|
34,6 ºC
|
20 ºC= 38,2 kPa // 25 ºC= 44,3 kPa // 30 ºC= 64,5 kPa // 35 ºC= 102,2 kPa
|
3
|
Propyl Acetate
|
C5H10O2
|
102 ºC
|
0 ºC= 4,33 kPa // 15 ºC= 7,83 kPa // 20 ºC= 9,22 kPa // 40 ºC= 10,07 kPa
|
4
|
Ethyl Acetate
|
C4H8O2
|
77,1 ºC
|
0 ºC= 9,46 kPa // 15 ºC= 12,18 kPa // 21 ºC= 14,41 kPa // 40 ºC= 20,07 kPa
|
5
|
2-Propanol
|
C3H8O
|
82,5 ºC
|
0 ºC= 0,53 kPa // 15 ºC= 1,84 kPa // 20 ºC= 3,50 kPa // 40 ºC= 8,84 kPa
|
6
|
Pentate
|
C5H12
|
309,2 ºC
|
0 ºC= 71,42 kPa // 15 ºC= 98,63 kPa // 20 ºC= 101,93 kPa // 35 ºC= 104,94 kPa
|
7
|
Methyl Acetate
|
C3H6O2
|
56,9 ºC
|
0 ºC= 0,89 kPa // 15 ºC= 9,39 kPa // 20 ºC= 20,59 kPa // 35 ºC= 21,93 kPa
|
As we see in the table, the chemical with the highest boiling point is pentate with 309ºC, followed by propyl acetate with 102ºC and 2-propanol with 82.5ºC.
It has the highest boiling point because it has a large amount of Waals forces as it is a
And we need more energy to separate the molecules. Pentane is
the only molecule which only has Van der Waals forces so has the lowest boiling
point.
COMPOUNDS:














Please include a short introduction.
ResponderEliminarGive your first logger pro graph a title to describe what it is showing.
Exellent table.
You need more detail explaining the boiling points of each chemical and how the boiling point is related to intermolecular forces. Remember, the more intermolecular forces a chemical has, the higher the boiling point.
Where are the references?
This is a good effort.
Formative B - 3 D - 4 E - 5
Done
ResponderEliminarSummative:
ResponderEliminarB - 4 Good effort but the blog could use more detail on relating the boiling points to intermolecular forces.
E - 6 The table and graph are well presented and well done for incuding a title for the graph.